Why do elements in the same group behave similarly
Home » Query » Why do elements in the same group behave similarlyYour Why do elements in the same group behave similarly images are available in this site. Why do elements in the same group behave similarly are a topic that is being searched for and liked by netizens today. You can Find and Download the Why do elements in the same group behave similarly files here. Download all royalty-free images.
If you’re searching for why do elements in the same group behave similarly images information linked to the why do elements in the same group behave similarly keyword, you have come to the right site. Our site always provides you with suggestions for seeking the highest quality video and picture content, please kindly surf and locate more enlightening video articles and graphics that match your interests.
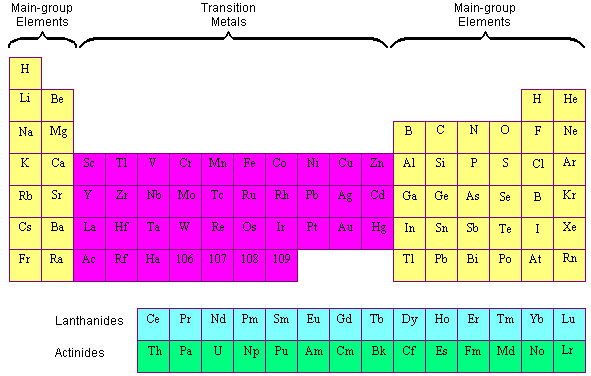
Why Do Elements In The Same Group Behave Similarly. A i All the elements of a group have similar chemical properties because they have same no. So these elements will show. Elements in the same group of the periodic table have similar properties because their electronic configurations have the same number of electrons in the outermost shell. Elements that are positioned in the same column or group of the periodic table exhibit similar properties.
 High School Chemistry Electron Configurations Of Main Group Elements Wikibooks Open Books For An Open World From en.wikibooks.org
High School Chemistry Electron Configurations Of Main Group Elements Wikibooks Open Books For An Open World From en.wikibooks.org
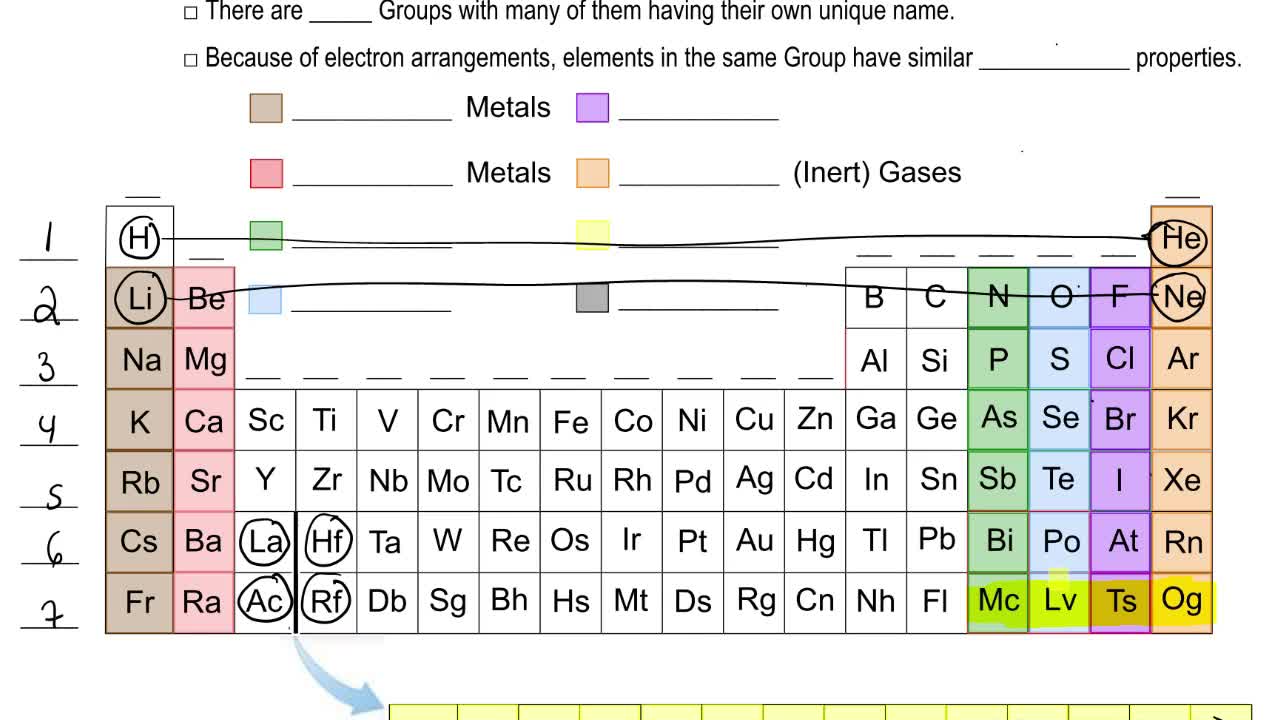
That is they have similar electron configurations. We do not mean to suggest that objective assessment of the environment is not also needed - it would be best to use objective and subjective. A i All the elements of a group have similar chemical properties because they have same no. Backtracking to explain valence electrons. Ii All the elements of a period have different chemical properties because they have different no. The properties of an element are largely determined by the valance electrons or outermost orbital.
This is because their atoms have the same number of electron in the highest occupied energy level.
We do not mean to suggest that objective assessment of the environment is not also needed - it would be best to use objective and subjective. D All of the above. Elements that are positioned in the same column or group of the periodic table exhibit similar properties. Since elements in the same group tend to react similarly they form only. All elements in a group have the same number of valence electrons. Transcribed image text.
 Source: chemed.chem.purdue.edu
Source: chemed.chem.purdue.edu
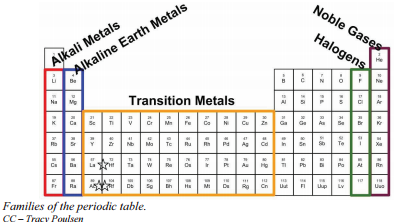
Elements in the same family column have similar properties because they have the same number of valence electrons which occur in higher energy levels. Elemental behavior is almost completely reliant on this outermost shell configuration with inner shells playing a less important role in determining properties. Elements that are positioned in the same column or group of the periodic table exhibit similar properties. Why do elements in the same group tend to have similar chemical properties. We do not mean to suggest that objective assessment of the environment is not also needed - it would be best to use objective and subjective.
 Source: pinterest.com
Source: pinterest.com
Both elements sodium Na and potassium K lie in the Group 1 column of the Periodic Table which contains the members of the Alkali Metals family. Elements in the group have similar chemical properties. Even if you dont want to stud. The elements of same group have similar chemical properties because they have same number of valence electrons. Since elements in the same group tend to react similarly they form isotopes with the same charge.
 Source: pinterest.com
Source: pinterest.com
Part of the reason is that they are in the same group in the periodic table and will have the same outer shell electron configuration. They are also both transition metals which also share similar properties. All elements in a group have the same number of valence electrons. Because of quantum mechanics electrons can only fit in certain fixed states around a nucleus the protons. Ii All the elements of a period have different chemical properties because they have different no.
 Source: pinterest.com
Source: pinterest.com
The connection here is that groups on the periodic table are organized according to valence electrons. C The number of electrons in their outermost shells is the same. All elements in a group have the same number of valence electrons. For example even if during home observations children in the same family appear to receive the same environmental treatment this does not mean that the children experienced the treatment similarly. The elements which belong to the same group will possess similarity in their number of available valence electrons.

We have studied how magnesium reacts with oxygen but calcium for example will behave in a similar way. Part of the reason is that they are in the same group in the periodic table and will have the same outer shell electron configuration. Ii All the elements of a period have different chemical properties because they have different no. This is because their atoms have the same number of electron in the highest occupied energy level. Transcribed image text.
 Source: study.com
Source: study.com
Of valence electrons in their atoms. Transcribed image text. C The number of electrons in their outermost shells is the same. B Within a group the atoms are the same size. You can watch the video in the visit link to confirm this.
 Source: britannica.com
Source: britannica.com
Of valence electrons in their outermost shell. The connection here is that groups on the periodic table are organized according to valence electrons. Even if you dont want to stud. These grades are the stepping stone to your future. Elements in the same group of the periodic table have similar properties because their electronic configurations have the same number of electrons in the outermost shell.
 Source: pinterest.com
Source: pinterest.com
When the periodic table was developed by Dimitri Mendeleev it was a result of him noticing reoccurring trends among the known elements at the time. These grades are the stepping stone to your future. Part of the reason is that they are in the same group in the periodic table and will have the same outer shell electron configuration. Magnesium is in group 2 in the Periodic Table. That is they have similar electron configurations.
 Source: pinterest.com
Source: pinterest.com
For example even if during home observations children in the same family appear to receive the same environmental treatment this does not mean that the children experienced the treatment similarly. C The number of electrons in their outermost shells is the same. We have studied how magnesium reacts with oxygen but calcium for example will behave in a similar way. Of valence electrons in their atoms. Because of quantum mechanics electrons can only fit in certain fixed states around a nucleus the protons.
 Source: pinterest.com
Source: pinterest.com
Since elements in the same group tend to react similarly they form only. B Within a group the atoms are the same size. A They all have the same number of protons. All elements in a group have the same number of valence electrons. These are the electrons which are in contact with the rest of the universe.
 Source: chem.libretexts.org
Source: chem.libretexts.org
That is they have similar electron configurations. Elements that are positioned in the same column or group of the periodic table exhibit similar properties. That is they have similar electron configurations. Since members of a period all have the same number of valance electrons they have similar properties and participate in similar reactions. Group 1 elements the alkali metals all have one electron i n their outer shell.
 Source: id.pinterest.com
Source: id.pinterest.com
Backtracking to explain valence electrons. Since elements in the same group tend to react similarly they form isotopes with the same charge. The properties of an element are largely determined by the valance electrons or outermost orbital. Ii All the elements of a period have different chemical properties because they have different no. These grades are the stepping stone to your future.
 Source: en.wikibooks.org
Source: en.wikibooks.org
So these elements will show. Li has 1 valence electron and so does N. As they are in the same group they have similar chemical properties and will react with substances including water in a. For example even if during home observations children in the same family appear to receive the same environmental treatment this does not mean that the children experienced the treatment similarly. A i All the elements of a group have similar chemical properties because they have same no.
 Source: clutchprep.com
Source: clutchprep.com
Ii All the elements of a period have different chemical properties because they have different no. Ii All the elements of a period have different chemical properties because they have different no. Backtracking to explain valence electrons. Since elements in the same group tend to react similarly they form only. Generally reactivity decreases as you go down the group in the periodic table.
 Source: pinterest.com
Source: pinterest.com
Both elements sodium Na and potassium K lie in the Group 1 column of the Periodic Table which contains the members of the Alkali Metals family. The elements in a group often look and behave similarly because they have the same number of electrons in their outermost shell the face they. The properties of an element are largely determined by the valance electrons or outermost orbital. Since elements in the same group tend to react similarly they form only. Of valence electrons in their outermost shell.
 Source: pinterest.com
Source: pinterest.com
When the periodic table was developed by Dimitri Mendeleev it was a result of him noticing reoccurring trends among the known elements at the time. Magnesium is in group 2 in the Periodic Table. Part of the reason is that they are in the same group in the periodic table and will have the same outer shell electron configuration. Generally reactivity decreases as you go down the group in the periodic table. Of valence electrons in their atoms.
 Source: nl.pinterest.com
Source: nl.pinterest.com
Elements that are positioned in the same column or group of the periodic table exhibit similar properties. Since members of a period all have the same number of valance electrons they have similar properties and participate in similar reactions. Group 1 elements the alkali metals all have one electron i n their outer shell. Why do elements in the same family have similar properties. All elements in a group have the same number of valence electrons.
 Source: britannica.com
Source: britannica.com
Magnesium is in group 2 in the Periodic Table. Since elements in the same group tend to react similarly they form isotopes with the same charge. Of valence electrons in their atoms. Ii All the elements of a period have different chemical properties because they have different no. Since members of a period all have the same number of valance electrons they have similar properties and participate in similar reactions.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title why do elements in the same group behave similarly by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.