Why do elements in a group behave similarly
Home » Query » Why do elements in a group behave similarlyYour Why do elements in a group behave similarly images are available in this site. Why do elements in a group behave similarly are a topic that is being searched for and liked by netizens now. You can Get the Why do elements in a group behave similarly files here. Download all free images.
If you’re looking for why do elements in a group behave similarly images information connected with to the why do elements in a group behave similarly interest, you have pay a visit to the right blog. Our website frequently provides you with suggestions for viewing the highest quality video and image content, please kindly search and locate more informative video articles and graphics that match your interests.
Why Do Elements In A Group Behave Similarly. Get an answer for Which group of elements will have similar chemical properties or reactivity. As you go down the groups though these atoms become more reactive and unstable because of the nucleus size. 5 Based on Table 1 we have a product line vector for each firm which has 10 elements taking the value 1 or 0. Elemental behavior is almost completely reliant on this outermost shell configuration with inner shells playing a less important role in determining properties.
 Premiere Pro Tip The Fx Badge Lets You Easily Identify If An Effect Has Been Applied To A Clip Gray Means No Effect Ap How To Apply Color Correction Let It From pinterest.com
Premiere Pro Tip The Fx Badge Lets You Easily Identify If An Effect Has Been Applied To A Clip Gray Means No Effect Ap How To Apply Color Correction Let It From pinterest.com
D All of the above. Group 1 compounds are more stable to heat than the corresponding compounds in Group 2. Get an answer for Which group of elements will have similar chemical properties or reactivity. One of the key insights behind the periodic table of the elements was that the chemical properties of elements tend to follow a repeating pattern. Components of social structure are culture social class social status roles groups and institutions. 5 Based on Table 1 we have a product line vector for each firm which has 10 elements taking the value 1 or 0.
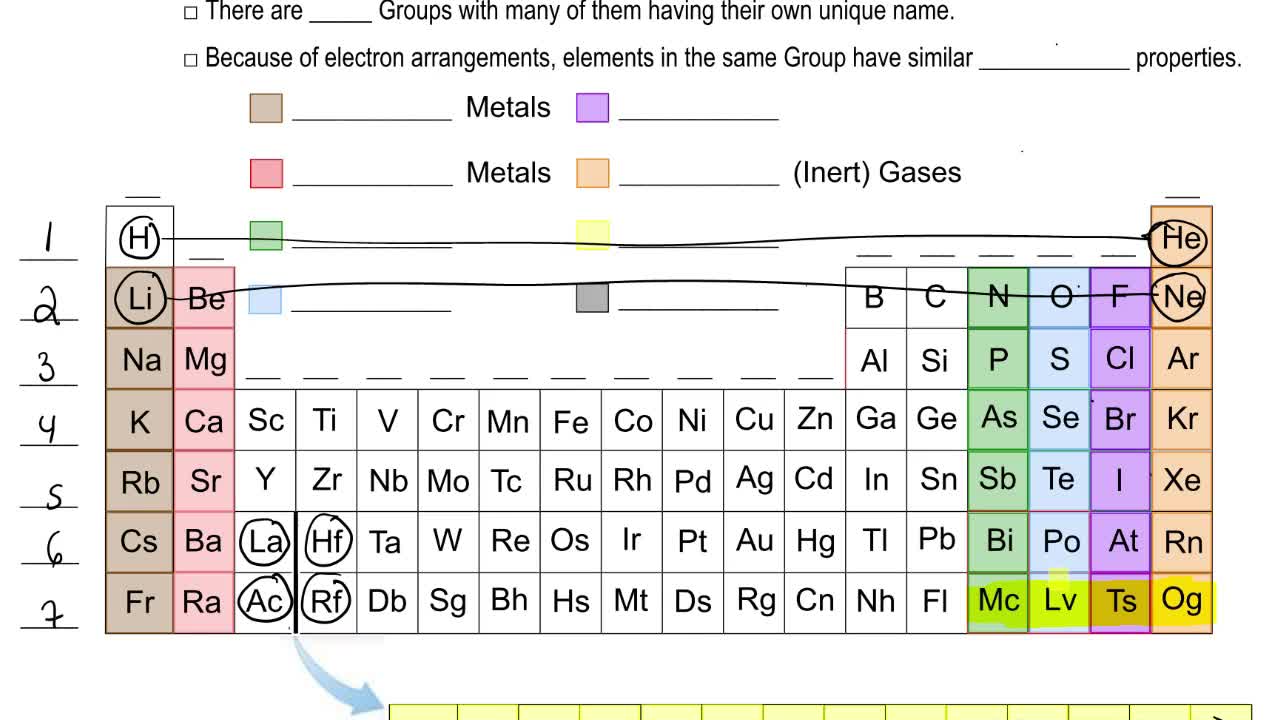
The elements of same group have similar chemical properties because they have same number of valence electrons.
Group 1 compounds are more stable to heat than the corresponding compounds in Group 2. Since members of a period all have the same number of valance electrons they have similar properties and participate in similar reactions. Get an answer for Which group of elements will have similar chemical properties or reactivity. One of the key insights behind the periodic table of the elements was that the chemical properties of elements tend to follow a repeating pattern. Of valence electrons in their atoms. Then we calculate the angle θ between each pair of vectors.
 Source: in.pinterest.com
Source: in.pinterest.com
Homomorphism from Greek homoios morphe similar form a special correspondence between the members elements of two algebraic systems such as two groups two rings or two fields. It includes the material objects used by a group. Two homomorphic systems have the same basic structure and while their elements and operations may appear entirely different results on one system often apply as well to the other system. For the general view these valance electrons are what allow atoms to react with one another. 5 Based on Table 1 we have a product line vector for each firm which has 10 elements taking the value 1 or 0.
 Source: clutchprep.com
Source: clutchprep.com
Group 12 by modern IUPAC numbering is a group of chemical elements in the periodic tableIt includes zinc Zn cadmium Cd and mercury Hg. An example would be the alkali. All the elements in one group have the same number of valence electrons. For the most part elements in the same group have the same number of valance electrons based on the average for stable isotopes. They are both FLUIDS and basically follow the same Laws of Physics.
 Source: en.wikibooks.org
Source: en.wikibooks.org
For the most part elements in the same group have the same number of valance electrons based on the average for stable isotopes. Of valence electrons in their outermost shell. An example would be the alkali. Li has 1 valence electron and so does N. Both elements sodium Na and potassium K lie in the Group 1 column of the Periodic Table which contains the members of the Alkali Metals family.
 Source: nl.pinterest.com
Source: nl.pinterest.com
Elements that are in the same column particularly the a group elements have similar. If two firms have the same product line cos θ has the maximum value which is equal to 1 and it decreases as the product lines of two firms become different. A They all have the same number of protons. Why do elements within a group family or column in the Periodic Table exhibit similar chemical behavior. B Within a group the atoms are the same size.
 Source: pinterest.com
Source: pinterest.com
For the general view these valance electrons are what allow atoms to react with one another. Part of the reason is that they are in the same group in the periodic table and will have the same outer shell electron configuration. Since members of a period all have the same number of valance electrons they have similar properties and participate in similar reactions. Since elements in a group have the same number of valence electrons they behave similarly in chemistry. Elements that are in the same column particularly the a group elements have similar.
 Source: pinterest.com
Source: pinterest.com
Part of the reason is that they are in the same group in the periodic table and will have the same outer shell electron configuration. Homomorphism from Greek homoios morphe similar form a special correspondence between the members elements of two algebraic systems such as two groups two rings or two fields. Part of the reason is that they are in the same group in the periodic table and will have the same outer shell electron configuration. It includes the material objects used by a group. C The number of electrons in their outermost shells is the same.
 Source: pinterest.com
Source: pinterest.com
Get an answer for Which group of elements will have similar chemical properties or reactivity. Similarly the atoms of all group 7 elements have similar chemical properties and reactions to each other because they all have seven electrons in their outer shell. The elements in a group often look and behave similarly because they have the same number of electrons in their outermost shell the face they. An example would be the alkali. You will often find that the lithium compounds behave similarly to Group 2 compounds but the rest of Group 1 are in some way different.
 Source: pinterest.com
Source: pinterest.com
It determines what kind of people we will become. Of valence electrons in their outermost shell. See full answer below. The biggest difference is that Liquids are NOT Compressible. If two firms have the same product line cos θ has the maximum value which is equal to 1 and it decreases as the product lines of two firms become different.
 Source: pinterest.com
Source: pinterest.com
One of the key insights behind the periodic table of the elements was that the chemical properties of elements tend to follow a repeating pattern. Of valence electrons in their atoms. The biggest difference is that Liquids are NOT Compressible. As you go down the groups though these atoms become more reactive and unstable because of the nucleus size. For the most part elements in the same group have the same number of valance electrons based on the average for stable isotopes.
 Source: pinterest.com
Source: pinterest.com
The valence electrons are those on the energy level the most distant from the nucleus. Of valence electrons in their atoms. Group 12 by modern IUPAC numbering is a group of chemical elements in the periodic tableIt includes zinc Zn cadmium Cd and mercury Hg. These are the electrons which are in contact with the rest of the universe. Since elements in a group have the same number of valence electrons they behave similarly in chemistry.
 Source: in.pinterest.com
Source: in.pinterest.com
The properties of an element are largely determined by the valance electrons or outermost orbital. Why gas and liquid behave similarly. The further inclusion of copernicium Cn in group 12 is supported by recent experiments on individual copernicium atoms. Elemental behavior is almost completely reliant on this outermost shell configuration with inner shells playing a less important role in determining properties. Components of social structure are culture social class social status roles groups and institutions.
 Source: pinterest.com
Source: pinterest.com
The valence electrons are those on the energy level the most distant from the nucleus. Elements in the same group of the periodic table have similar properties because their electronic configurations have the same number of electrons in the outermost shell. One of the key insights behind the periodic table of the elements was that the chemical properties of elements tend to follow a repeating pattern. These valence electrons are those involved in bonding with other atoms to form compounds. You will often find that the lithium compounds behave similarly to Group 2 compounds but the rest of Group 1 are in some way different.
 Source: in.pinterest.com
Source: in.pinterest.com
It determines what kind of people we will become. Group 12 by modern IUPAC numbering is a group of chemical elements in the periodic tableIt includes zinc Zn cadmium Cd and mercury Hg. Group 1 compounds are more stable to heat than the corresponding compounds in Group 2. The elements of same group have similar chemical properties because they have same number of valence electrons. The further inclusion of copernicium Cn in group 12 is supported by recent experiments on individual copernicium atoms.
 Source: pinterest.com
Source: pinterest.com
Then we calculate the angle θ between each pair of vectors. It includes the material objects used by a group. All the elements in one group have the same number of valence electrons. Components of social structure are culture social class social status roles groups and institutions. Both elements sodium Na and potassium K lie in the Group 1 column of the Periodic Table which contains the members of the Alkali Metals family.
 Source: pinterest.com
Source: pinterest.com
For the general view these valance electrons are what allow atoms to react with one another. Elemental behavior is almost completely reliant on this outermost shell configuration with inner shells playing a less important role in determining properties. The biggest difference is that Liquids are NOT Compressible. Since members of a period all have the same number of valance electrons they have similar properties and participate in similar reactions. Ii All the elements of a period have different chemical properties because they have different no.
 Source: pinterest.com
Source: pinterest.com
Part of the reason is that they are in the same group in the periodic table and will have the same outer shell electron configuration. Formerly this group was named IIB pronounced as group two B as the II is a Roman numeral by CAS and old IUPAC system. Why do elements within a group family or column in the Periodic Table exhibit similar chemical behavior. C The number of electrons in their outermost shells is the same. As you go down the groups though these atoms become more reactive and unstable because of the nucleus size.
 Source: pinterest.com
Source: pinterest.com
The effect of heat on Group 1 compounds. Both elements sodium Na and potassium K lie in the Group 1 column of the Periodic Table which contains the members of the Alkali Metals family. Why gas and liquid behave similarly. The effect of heat on Group 1 compounds. The elements of same group have similar chemical properties because they have same number of valence electrons.
 Source: pinterest.com
Source: pinterest.com
Homomorphism from Greek homoios morphe similar form a special correspondence between the members elements of two algebraic systems such as two groups two rings or two fields. When you put the elements in order by number of protons and then line up the elements in rows with similar elements places in columns togethe. Group 12 by modern IUPAC numbering is a group of chemical elements in the periodic tableIt includes zinc Zn cadmium Cd and mercury Hg. Of valence electrons in their atoms. All the elements in one group have the same number of valence electrons.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title why do elements in a group behave similarly by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.