The second law of thermodynamics states that _____
Home » Query » The second law of thermodynamics states that _____Your The second law of thermodynamics states that _____ images are ready in this website. The second law of thermodynamics states that _____ are a topic that is being searched for and liked by netizens now. You can Get the The second law of thermodynamics states that _____ files here. Find and Download all free photos and vectors.
If you’re searching for the second law of thermodynamics states that _____ pictures information connected with to the the second law of thermodynamics states that _____ topic, you have visit the right site. Our site frequently provides you with suggestions for refferencing the highest quality video and image content, please kindly search and locate more informative video content and graphics that fit your interests.
The Second Law Of Thermodynamics States That _____. The Journal of Philosophy. According to the second law of thermodynamics the whole heat energy cannot be converted into work and part of the energy must be rejected to the surroundings. The Second Law of Thermodynamics is about the quality of energy. Second Law- In a natural thermodynamic process the sum of the entropies of the interacting thermodynamic systems increases.
 Second Law Of Thermodynamics An Overview Sciencedirect Topics From sciencedirect.com
Second Law Of Thermodynamics An Overview Sciencedirect Topics From sciencedirect.com
The second law states that if the physical process is irreversible the combined entropy of the system and the environment must increase. A that spontaneous processes are characterized by the overall conversion of order to disorder. The second law of thermodynamics states that the heat energy cannot transfer from a body at a lower temperature to a body at a higher temperature without the addition of energy. State the Second Law of Thermodynamics. Second Law of Thermodynamics - Increased Entropy The Second Law of Thermodynamics is commonly known as the Law of Increased Entropy. Ultimately this is one of the key elements dictating an.
It states that the change in a.
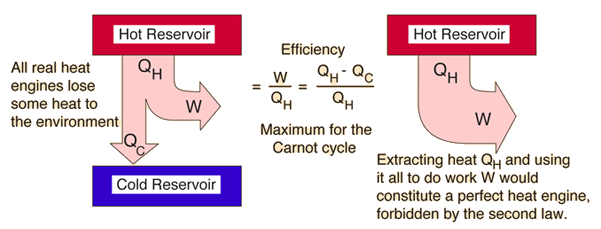
B that spontaneous processes are characterized by the conversion of work to force. The figure below shows the possible machine in which heat is supplied from the hot reservoir work is done on the surroundings and remaining is rejected to cool reservoir mostly the atmosphere. The second law of thermodynamics states that you can move heat from a hotter place to a colder place without doing work but that you need to work to move heat from a colder place to a hotter place. The second law of thermodynamics states. Second Law of Thermodynamics - Increased Entropy The Second Law of Thermodynamics is commonly known as the Law of Increased Entropy. The Second Law of Thermodynamics relates the concept of entropy a measure of disorder to the concepts of heat and temperature.

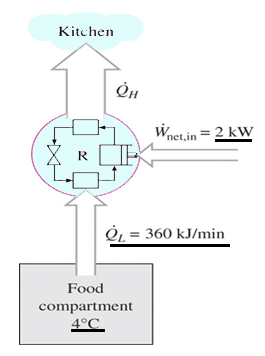
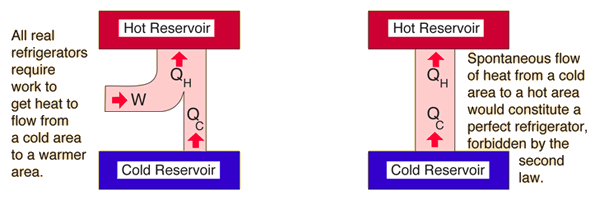
The second law of thermodynamics states that you can move heat from a hotter place to a colder place without doing work but that you need to work to move heat from a colder place to a hotter place. A spontaneous process is a process that occurs in a system without any input of energy from the surroundings. The entropy of the universe must always increase for an spontaneous reaction. This is why running an air conditioner for a long period of time costs you money. A that spontaneous processes are characterized by the overall conversion of order to disorder.
 Source: energyeducation.ca
Source: energyeducation.ca
While quantity remains the same First Law the quality of matterenergy deteriorates gradually over time. The entropy of the universe must always increase for an spontaneous reaction. 8 The Second Law of Thermodynamics tells us that everything in the universe is slowly collapsing into disorder. According to the second law of thermodynamics the whole heat energy cannot be converted into work and part of the energy must be rejected to the surroundings. B that spontaneous processes are characterized by the conversion of work to force.
 Source: sciencedirect.com
Source: sciencedirect.com
Second Law of Thermodynamics - Increased Entropy The Second Law of Thermodynamics is commonly known as the Law of Increased Entropy. In philosophy of physics. 8 The Second Law of Thermodynamics tells us that everything in the universe is slowly collapsing into disorder. A that spontaneous processes are characterized by the overall conversion of order to disorder. The second law of thermodynamics states that entropy which is often thought of as simple disorder will always increase within a closed system.
 Source: ohio.edu
Source: ohio.edu
A that spontaneous processes are characterized by the overall conversion of order to disorder. The second law of thermodynamics states that you can move heat from a hotter place to a colder place without doing work but that you need to work to move heat from a colder place to a hotter place. Maxwell as a counter-example of the second law of thermodynamics that is a perpetuum mobile of the second kind. The entropy of the universe must always increase for an spontaneous reaction. The second law of thermodynamics states that the heat energy cannot transfer from a body at a lower temperature to a body at a higher temperature without the addition of energy.
 Source: examhill.com
Source: examhill.com
State the Second Law of Thermodynamics. The second law states that if the physical process is irreversible the combined entropy of the system and the environment must increase. The final entropy must be greater than the initial entropy for an irreversible process. What is the second law of thermodynamics in simple terms. In philosophy of physics.
 Source: sanfoundry.com
Source: sanfoundry.com
What is the second law of thermodynamics in simple terms. In philosophy of physics. Second Law- In a natural thermodynamic process the sum of the entropies of the interacting thermodynamic systems increases. It states that the change in a. This is why running an air conditioner for a long period of time costs you money.
 Source: energyeducation.ca
Source: energyeducation.ca
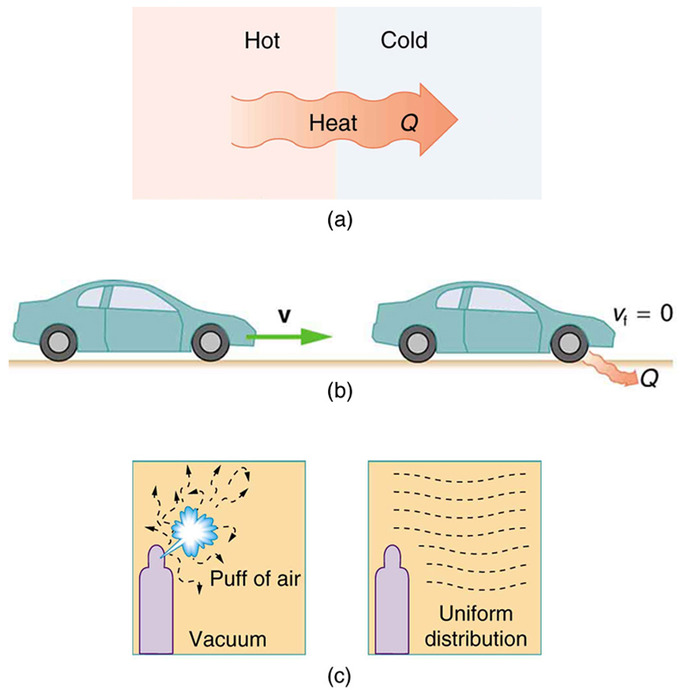
The second law of thermodynamics states that entropy which is often thought of as simple disorder will always increase within a closed system. It states that as energy is transferred or transformed more and more of it is wasted. Maxwell as a counter-example of the second law of thermodynamics that is a perpetuum mobile of the second kind. While quantity remains the same First Law the quality of matterenergy deteriorates gradually over time. A spontaneous process is a process that occurs in a system without any input of energy from the surroundings.
 Source: toppr.com
Source: toppr.com
The entropy of the universe must always increase for an spontaneous reaction. The second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous process. In simple words the law explains that an isolated systems entropy will never decrease over time. The second law of thermodynamics states that you can move heat from a hotter place to a colder place without doing work but that you need to work to move heat from a colder place to a hotter place. It states that as energy is transferred or transformed more and more of it is wasted.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Second Law of Thermodynamics - Increased Entropy The Second Law of Thermodynamics is commonly known as the Law of Increased Entropy. Maxwell as a counter-example of the second law of thermodynamics that is a perpetuum mobile of the second kind. The second law of thermodynamics states that By signing up youll get thousands of step-by-step solutions to your homework questions. 1 Application of the second law of thermodynamics helps explain the various ways in which engines transform heat into mechanical work as for instance in the gasoline engine of a car or in a steam. It states that as energy is transferred or transformed more and more of it is wasted.

In philosophy of physics. The second law of thermodynamics states that the total entropy can only increase over time for an isolated system meaning a system which neither energy nor matter can enter or leave. Ultimately this is one of the key elements dictating an. The second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous process. While quantity remains the same First Law the quality of matterenergy deteriorates gradually over time.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The entropy of the universe must always increase for an spontaneous reaction. The second law of thermodynamics states that By signing up youll get thousands of step-by-step solutions to your homework questions. Second Law- In a natural thermodynamic process the sum of the entropies of the interacting thermodynamic systems increases. An important implication of this law is that heat transfers energy spontaneously from higher- to lower-temperature objects but never spontaneously in the reverse direction. It states that as energy is transferred or transformed more and more of it is wasted.
 Source: ohio.edu
Source: ohio.edu
It states that as energy is transferred or transformed more and more of it is wasted. The Journal of Philosophy. A spontaneous process is a process that occurs in a system without any input of energy from the surroundings. In philosophy of physics. A that spontaneous processes are characterized by the overall conversion of order to disorder.
 Source: sciencedirect.com
Source: sciencedirect.com
In philosophy of physics. The figure below shows the possible machine in which heat is supplied from the hot reservoir work is done on the surroundings and remaining is rejected to cool reservoir mostly the atmosphere. The second law of thermodynamics tells us which state transformations are so statistically unlikely that they are effectively forbidden. The Second Law of Thermodynamics states that in all energy exchanges if no energy enters or leaves the system the potential energy of the state will always be less than that of the initial state This is also commonly referred to as entropy. B that spontaneous processes are characterized by the conversion of work to force.
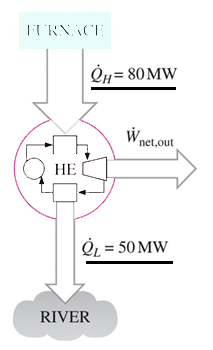 Source: sanfoundry.com
Source: sanfoundry.com
The second law of thermodynamics states that the total entropy can only increase over time for an isolated system meaning a system which neither energy nor matter can enter or leave. The second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous process. A that spontaneous processes are characterized by the overall conversion of order to disorder. What is the second law of thermodynamics in simple terms. The Second Law also states that there is a natural tendency of any isolated system to degenerate into a more disordered state22 мая 2015 г.
 Source: ohio.edu
Source: ohio.edu
The figure below shows the possible machine in which heat is supplied from the hot reservoir work is done on the surroundings and remaining is rejected to cool reservoir mostly the atmosphere. B that spontaneous processes are characterized by the conversion of work to force. Its original formulation due to Clausius states that Heat can never pass from a colder to a warmer body without some other change connected therewith occurring at the same time. In simple words the law explains that an isolated systems entropy will never decrease over time. The final entropy must be greater than the initial entropy for an irreversible process.
 Source: ohio.edu
Source: ohio.edu
Its original formulation due to Clausius states that Heat can never pass from a colder to a warmer body without some other change connected therewith occurring at the same time. What is the second law of thermodynamics in simple terms. It states that as energy is transferred or transformed more and more of it is wasted. The second law of thermodynamics states that the total entropy of an isolated system the thermal energy per unit temperature that is unavailable for doing useful work can never decrease. The Second Law of Thermodynamics relates the concept of entropy a measure of disorder to the concepts of heat and temperature.
 Source: clutchprep.com
Source: clutchprep.com
It states that the change in a. The entropy of the universe must always increase for an spontaneous reaction. The Second Law of Thermodynamics states that in all energy exchanges if no energy enters or leaves the system the potential energy of the state will always be less than that of the initial state This is also commonly referred to as entropy. It states that as energy is transferred or transformed more and more of it is wasted. It states that as energy is transferred or transformed more and more of it is wasted.
 Source: energyeducation.ca
Source: energyeducation.ca
The Second Law also states that there is a natural tendency of any isolated system to degenerate into a more disordered state22 мая 2015 г. The second law of thermodynamics states that the total entropy of an isolated system the thermal energy per unit temperature that is unavailable for doing useful work can never decrease. A that spontaneous processes are characterized by the overall conversion of order to disorder. In philosophy of physics. 1 Application of the second law of thermodynamics helps explain the various ways in which engines transform heat into mechanical work as for instance in the gasoline engine of a car or in a steam.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title the second law of thermodynamics states that _____ by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.