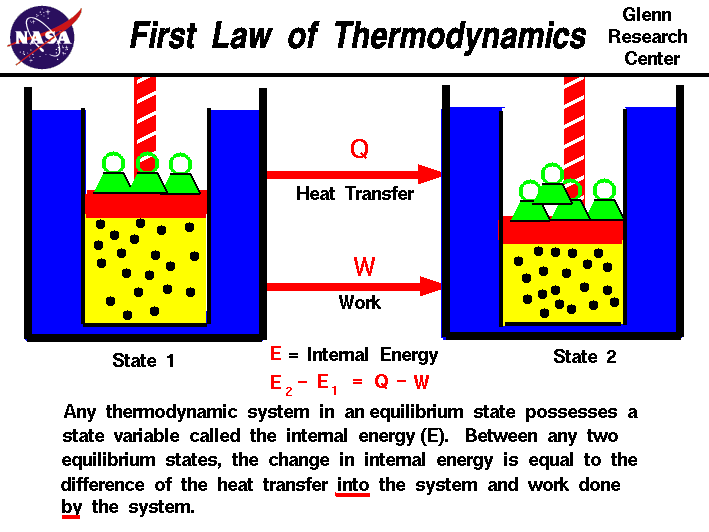
The first law of thermodynamics states that _____
Home » Query » The first law of thermodynamics states that _____Your The first law of thermodynamics states that _____ images are ready in this website. The first law of thermodynamics states that _____ are a topic that is being searched for and liked by netizens now. You can Download the The first law of thermodynamics states that _____ files here. Get all royalty-free photos.
If you’re searching for the first law of thermodynamics states that _____ pictures information connected with to the the first law of thermodynamics states that _____ interest, you have visit the right site. Our site frequently provides you with hints for seeing the maximum quality video and picture content, please kindly surf and locate more enlightening video articles and images that fit your interests.
The First Law Of Thermodynamics States That _____. The First Law of Thermodynamics states that energy cannot be created or destroyed. Although the definition seems very technical and challenging to understand numerous everyday examples apply this thermodynamic principle. First law of thermodynamics. In equation form the first law of thermodynamics is ΔU Q W.
 Quiz Worksheet First Law Of Thermodynamics Applied Study Com From study.com
Quiz Worksheet First Law Of Thermodynamics Applied Study Com From study.com
First Law of Thermodynamics. In equation form the first law of thermodynamics is ΔU Q W. The second law of thermodynamics states that the entropy of any isolated system always increases. System can do work. In other words the total energy of the universe remains constant. The classical form of the law is the following equation.
The First Law of Thermodynamics states that heat is a form of energy and thermodynamic processes are therefore subject to the principle of conservation of energy.
According to First law of thermodynamics. The first law of thermodynamics can be applied to the Cyclic and Non-Cyclic processes. First Law of Thermodynamics. This means that heat energy cannot be created or destroyed. It can however be transferred from one location to another and converted to and from other forms of energy. I Closed System Cyclic Process - It states that if a system executes a cycle transferring work and heat through its boundary the net heat transfer is equivalent to the network transfer and does not place any restriction on the direction of flow but the reversal of the process not violet the first law.
 Source: slidetodoc.com
Source: slidetodoc.com
The first law of thermodynamics can be applied to the Cyclic and Non-Cyclic processes. It says that all inputs and outputs of energy should add up to the total change in the energy of a system. Energy can cross the boundaries of a closed system in the form of heat or work. Energy transfer across a system boundary due solely to the temperature difference between a system and its surroundings is called heat. The First Law of Thermodynamics states that the internal energy of a thermodynamic system tends to increase when heat is supplied to the system from the surrounding and it tends to decrease when.

This means that heat energy cannot be created or destroyed. Energy can cross the boundaries of a closed system in the form of heat or work. Well assume the piston can move up and down compressing the gas or allowing the gas to expand but no. The first law of thermodynamics is also called as law of conservation of energy. According to First law of thermodynamics.

The _____ states that the increase in the internal energy of thermodynamic system is equal to the amount of heat energy added to the system minus the work done by the system on the surroundings. The First Law of Thermodynamics The first law of thermodynamics is an expression of the conservation of energy principle. System can do work. It says that all inputs and outputs of energy should add up to the total change in the energy of a system. It can only be converted from one form to another.
 Source: slidetodoc.com
Source: slidetodoc.com
In this equation dW. First law of thermodynamics. The first law of thermodynamics states that the change in internal energy of a system equals the net heat transfer into the system minus the net work done by the system. Brayton cycle or Rankine cycle. The first law of thermodynamics in terms of enthalpy show us why engineers use the enthalpy in thermodynamic cycles eg.
 Source: examhill.com
Source: examhill.com
The First Law of Thermodynamics The first law of thermodynamics is an expression of the conservation of energy principle. Although the definition seems very technical and challenging to understand numerous everyday examples apply this thermodynamic principle. Who made first law of thermodynamics. Heat is a form of energy. The First Law of Thermodynamics states that energy cannot be created or destroyed.
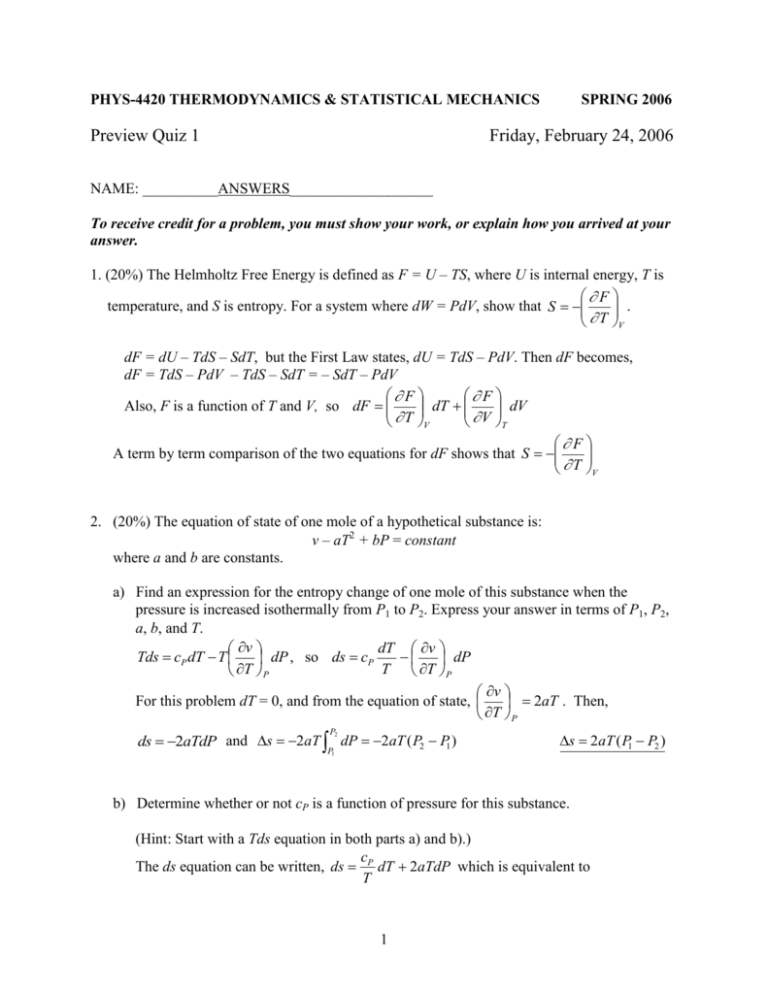 Source: studylib.net
Source: studylib.net
It can only be converted from one form to another. Brayton cycle or Rankine cycle. Well assume the piston can move up and down compressing the gas or allowing the gas to expand but no. The classical form of the law is the following equation. According to First law of thermodynamics.
 Source: slidetodoc.com
Source: slidetodoc.com
In this equation dW. System can do work. Nothing quite exemplifies the first law of thermodynamics as well as a gas like air or helium trapped in a container with a tightly fitting movable piston as seen below. DU dQ dW. The first law of thermodynamics can be applied to the Cyclic and Non-Cyclic processes.
This means that heat energy cannot be created or destroyed. The First Law of Thermodynamics The first law of thermodynamics is an expression of the conservation of energy principle. In this equation dW. The first law of thermodynamics states. According to First law of thermodynamics.
 Source: study.com
Source: study.com
First law of thermodynamics states that. Correct option is. DU dQ dW. System can do work. The first law of thermodynamics states that.

Well assume the piston can move up and down compressing the gas or allowing the gas to expand but no. Well assume the piston can move up and down compressing the gas or allowing the gas to expand but no. The First Law is used to categorise the performance of cyclic conversion systems like fossil-fired steam power cycles or geothermal cycles. The First Law of Thermodynamics The first law of thermodynamics is an expression of the conservation of energy principle. Who made first law of thermodynamics.
 Source: slidetodoc.com
Source: slidetodoc.com
The First Law of Thermodynamics states that energy can be converted from one form to another with the interaction of heat work and internal energy but it cannot be created nor destroyed under any circumstances. Well assume the piston can move up and down compressing the gas or allowing the gas to expand but no. You can think of the first law of thermodynamics as an energy accounting method. This means that heat energy cannot be created or destroyed. The first law also known as Law of Conservation of Energy states that energy cannot be created or destroyed in an isolated system.
 Source: slidetodoc.com
Source: slidetodoc.com
Well assume the piston can move up and down compressing the gas or allowing the gas to expand but no. This means that heat energy cannot be created or destroyed. Although the definition seems very technical and challenging to understand numerous everyday examples apply this thermodynamic principle. The first law asserts that if heat is recognized as a form of energy then the total energy of a system plus its surroundings is conserved. Heat is a form of energy.
 Source: studylib.net
Source: studylib.net
The first law of thermodynamics can be applied to the Cyclic and Non-Cyclic processes. The classical form of the law is the following equation. Who made first law of thermodynamics. In other words the total energy of the universe remains constant. The First Law is used to categorise the performance of cyclic conversion systems like fossil-fired steam power cycles or geothermal cycles.
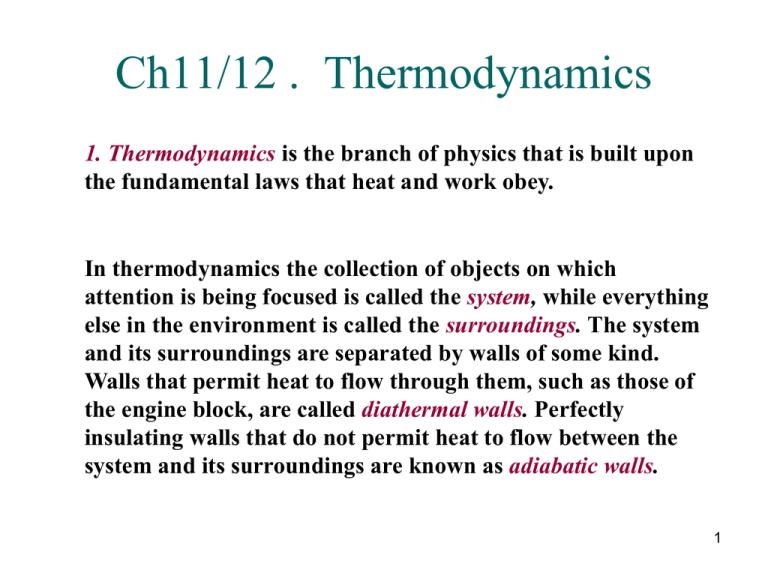 Source: studylib.net
Source: studylib.net
In equation form the first law of thermodynamics is ΔU Q W. The first law of thermodynamics in terms of enthalpy show us why engineers use the enthalpy in thermodynamic cycles eg. This means that heat energy cannot be created or destroyed. Who made first law of thermodynamics. First Law of Thermodynamics.

Mathematically this is represented as. Brayton cycle or Rankine cycle. The first law of thermodynamics states that. First Law of Thermodynamics. Heat is a form of energy.

The First Law is used to categorise the performance of cyclic conversion systems like fossil-fired steam power cycles or geothermal cycles. The first law also known as Law of Conservation of Energy states that energy cannot be created or destroyed in an isolated system. First Law of Thermodynamics. The total energy of an isolated system is neither created nor destroyed the amount of energy remains constant Energy is transformed from one form to another. Correct option is.
 Source: slidetodoc.com
Source: slidetodoc.com
The First Law of Thermodynamics The first law of thermodynamics is an expression of the conservation of energy principle. This means that heat energy cannot be created or destroyed. The first law also known as Law of Conservation of Energy states that energy cannot be created or destroyed in an isolated system. Brayton cycle or Rankine cycle. We will use three examples.
 Source: slidetodoc.com
Source: slidetodoc.com
Correct option is. In this equation dW. System can do work. The First Law of Thermodynamics states that heat is a form of energy and thermodynamic processes are therefore subject to the principle of conservation of energy. Lets discuss the first law of thermodynamics to a cyclic process and is as follows.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title the first law of thermodynamics states that _____ by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.